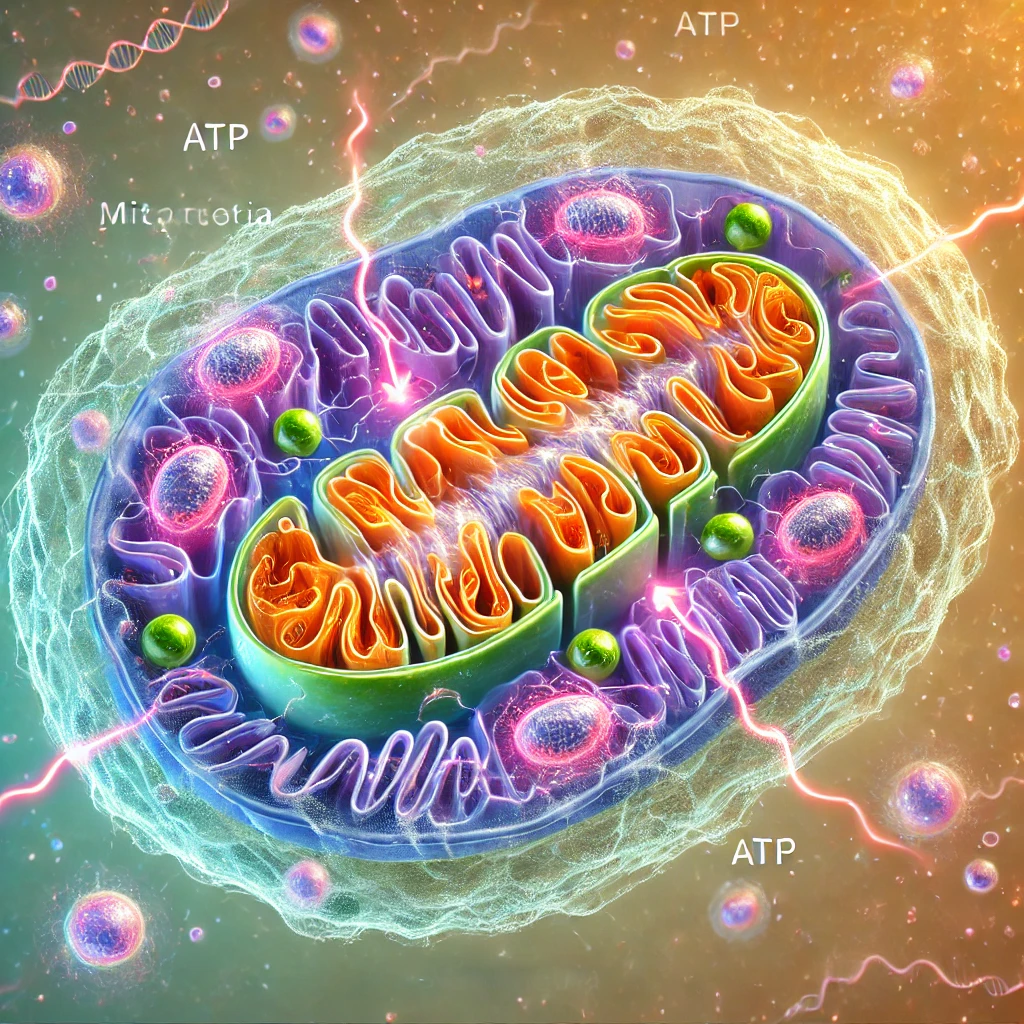
One of the most underappreciated insights in biology is that the cells of complex life are really tiny collaborations between formerly separate organisms. At the heart of every human cell—indeed, every animal, plant, and fungal cell—resides a tiny structure known as a mitochondrion. These mitochondria generate much of the cell’s energy, but what’s truly remarkable is their origin story: they are the evolutionary descendants of ancient free-living bacteria that were engulfed by a larger host cell more than a billion years ago.
This wasn’t a case of a cell simply “digesting” another organism. Instead, the absorbed bacteria stuck around, gradually evolving into crucial cellular residents over countless generations. They kept their own DNA, separate from the cell’s main genetic material, and continued to carry out specialized tasks. By outsourcing energy production to mitochondria, the host cells gained unprecedented power to fuel more complex operations, enabling the evolution of larger, multicellular life forms—plants, animals, and eventually us.
What makes this doubly fascinating is that these ancient partnerships still shape our health and behavior. Mitochondrial genes are passed down almost exclusively from the mother’s egg cell, meaning your mitochondrial DNA forms a maternal genetic lineage connecting you to ancestors long past. Without these ancient symbiotic mergers, life as we know it—complex organisms made of trillions of cooperative cells—likely wouldn’t exist. It’s a subtle reminder that fundamental parts of our biology are built on unexpected alliances forged deep in evolutionary time.
References
Margulis, L. (1970). Origin of Eukaryotic Cells.
Yale University Press.Lane, N. (2005). Power, Sex, Suicide: Mitochondria and the Meaning of Life. Oxford University Press.